Center for Medical Freedom
(a project of CLDEF)
FDA Bureaucrats Seek to Restrict Access to Homeopathic Medicines
… And what we can do to STOP THEM.
If you don’t have the time to read this entire article, but you understand the importance of continued access to Homeopathic Remedies — please go right to the end of this article and file your comments with the FDA.
![<a title="By The U.S. Food and Drug Administration (FDA Sign & Bldg 21 at Entrance) [Public domain], via Wikimedia Commons"</a>](http://cldef.org/wordpress/wp-content/uploads/2018/03/FDA_Sign-300x264.jpg) to take a fresh look at restricting access to Homeopathic remedies and products. Is that a good enough reason to clamp down on Homeopathy? While there have been charges made about the safety of a few Homeopathic products (e.g., Belladonna and Homeopathic teething tablets), these charges have been shown to be baseless, and can usually be tracked back to Big Pharma and its minions. Absolutely no evidence has been presented that any of these charges against these products are true, and significant proof should be demanded before such charges are believed and acted upon. The few specific complaints the FDA points to must be carefully analyzed for other causative factors. Some of those could include whether the babies that reportedly developed seizures had recently been vaccinated, or were given other drug products, or were nursing mothers taking dangerous, but FDA-approved, pharmaceutical drugs.
to take a fresh look at restricting access to Homeopathic remedies and products. Is that a good enough reason to clamp down on Homeopathy? While there have been charges made about the safety of a few Homeopathic products (e.g., Belladonna and Homeopathic teething tablets), these charges have been shown to be baseless, and can usually be tracked back to Big Pharma and its minions. Absolutely no evidence has been presented that any of these charges against these products are true, and significant proof should be demanded before such charges are believed and acted upon. The few specific complaints the FDA points to must be carefully analyzed for other causative factors. Some of those could include whether the babies that reportedly developed seizures had recently been vaccinated, or were given other drug products, or were nursing mothers taking dangerous, but FDA-approved, pharmaceutical drugs.One has to question the FDA’s recent assertions that some Homeopathic remedies pose “risk” requiring additional regulation, considering the 200-plus year track record of safety of Homeopathy. Moreover, it should be understood that the FDA receives three-quarters of its funding from pharmaceutical drug companies and has a known history of corruption. (See, e.g., “FDA Depends on Industry Funding; Money Comes with “Strings Attached.”)
Homeopathic product sales have been surging, not due to consumer ignorance, as the FDA assumes, but the opposite – more informed consumers are opting for safer, gentler medicine over pharmaceutical chemicals. A survey done by Mass. General Hospital, as reported in the American Journal of Public Health (Feb 18, 2016), found that users of Homeopathic products were more likely to be “highly educated.” For 200 years, Homeopathic remedies have survived attempts by proponents of pharmaceutical drugs to suppress Homeopathy time and again, but the threat here is real and must be taken seriously.
My intent here is not to convince anyone of the superiority of Homeopathy to allopathic drugs, or even of its efficacy for all, but to insist that the federal law that has protected the public access to Homeopathic remedies for 79 years not be ignored by unelected bureaucrats. It is not the job of FDA employees to place their value judgment on this system of medicine, but only to ensure that such drugs are manufactured according to legal standards and are properly labeled.
Federal drug law was initially designed to ensure that any drug product sold would be unadulterated, and its ingredients safe and fully disclosed. The Pure Food and Drugs Act was enacted in 1906 (named “FDA” in 1930), to prohibit misbranded and adulterated foods and drugs from interstate commerce. The demand for stricter FDA oversight of drugs was fueled by the “Sulfanilamide Disaster” of 1937, when a liquid antibiotic poisoned and killed over 100 people as they unknowingly ingested diethylene glycol, an untested, toxic diluent used in the preparation of the drug. Hence the Food Drug and Cosmetic Act of 1938 (FDCA) was enacted to give the FDA responsibility for the safety of drugs, food, and cosmetics. However, that law recognized that Homeopathy was very different from toxic pharmaceutical drugs, and it wrote that difference into the law.
The principal author of the FDCA, U.S. Senator Royal S. Copeland (D-NY), was himself a homeopath (and an ophthalmologist), and thus fully understood the nature of Homeopathy, calling it a distinct, but not inferior, medicine. That distinction was enacted into law.
Unlike Homeopathic remedies, pharmaceutical drugs are basically chemicals, and all have a level at which they become toxic. Pharmaceutical drugs taken orally are metabolized by the body, causing a chemical reaction that hopefully will achieve its primary purpose, hopefully with fewer adverse side effects than benefits. Homeopathic drugs are quite different. They could best be described as dynamic, not chemical. They are not metabolized by the body, and exert no chemical action within or on it.
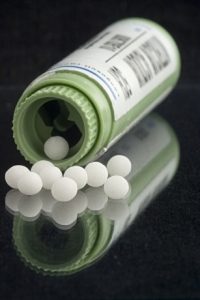 A true Homeopathic remedy is made from a tincture that has been diluted beyond Avogadro’s scale, meaning not a single molecule of the original substance remains. (Most Homeopathic remedies are made by “medicating” sugar pellets with such super-dilution, but some are left in liquid form.) Skeptics mock that a Homeopathic drug can’t do anything since it is “nothing,” and insist that any good result from homeopathy can only be due to placebo effect. However, even if that were right, their use cannot be dangerous, and should not be prohibited.
A true Homeopathic remedy is made from a tincture that has been diluted beyond Avogadro’s scale, meaning not a single molecule of the original substance remains. (Most Homeopathic remedies are made by “medicating” sugar pellets with such super-dilution, but some are left in liquid form.) Skeptics mock that a Homeopathic drug can’t do anything since it is “nothing,” and insist that any good result from homeopathy can only be due to placebo effect. However, even if that were right, their use cannot be dangerous, and should not be prohibited.
However, the fact is that Homeopathic drugs work, being energetic in operation, which is what makes them fundamentally safe. They present absolutely no danger of toxicity, and have no side-effects. Any perceived “side-effects” from a Homeopathic medicine can only be the body’s natural response to its energy, while the body heals itself, and are always transient and mild. The healing effect from a Homeopathic remedy comes about by triggering the body’s energy to heal itself, just the way God designed it. Granted, this may be difficult for the unelected bureaucrats at the FDA to understand or accept, but people all over the world have discovered the gentle effectiveness of Homeopathy for themselves, experientially, for over 200 years.
For all these reasons, Homeopathic products cannot be properly judged by the same tests used for toxic chemical drugs. Therefore, under Sections 201(g) and 201(j) of the FDCA, Congress mandated that Homeopathic drugs “shall be subject to the provisions of the Homeopathic Pharmacopeia.” Since 1938, Homeopathic preparations have been regulated and protected by this law, and such medications have been formulated according to provisions of the Homeopathic Pharmacopeia of the United States (HPUS), which the Act recognizes as an official drug compendium of monographs (listings of drug data).
It is a principle and law of Homeopathy as established by founder Dr. Samuel Christian Hahnemann, that any Homeopathic ![<a title="By ?? (??) [Public domain], via Wikimedia Commons"</a>](http://cldef.org/wordpress/wp-content/uploads/2018/03/Samuel_Hahnemann_Monument-300x216.jpg) remedy first be extensively tested by way of drug “provings.” In these tests, a pharmacologically active substance is given to healthy people – to see what symptoms it creates – and then a Homeopathically prepared dose of the same is given to people with those symptoms – to see what symptoms the remedy can mitigate. Innumerable compilations of these Homeopathic provings in book form provide detailed clinical confirmation of their usefulness.
remedy first be extensively tested by way of drug “provings.” In these tests, a pharmacologically active substance is given to healthy people – to see what symptoms it creates – and then a Homeopathically prepared dose of the same is given to people with those symptoms – to see what symptoms the remedy can mitigate. Innumerable compilations of these Homeopathic provings in book form provide detailed clinical confirmation of their usefulness.
The HPUS has been in continuous publication since 1841. In 1980, the Homeopathic Pharmacopeia Convention of the United States was formed as a standard-setting organization, to focus on the regulatory approval of Homeopathic remedies and the development and publication of general pharmacy practices and standards. The criteria for inclusion in the HPUS require that a Homeopathic remedy be determined by HPCUS to be safe and effective and to be prepared according to the specifications of the HPUS general pharmacy section. Rather than following the new-drug approval process, premarket approval for new Homeopathic remedies is accomplished by way of review and “monograph approval” by the HPCUS.
Many of the most frequently used Homeopathic remedies used today were listed in the original HPUS in 1938 and should therefore be automatically protected from bureaucratic meddling. Nevertheless, now the FDA, acting on behalf of Big Pharma, seeks to consider Homeopathic remedies “new drugs,” ignoring the fact that they were “subject to the Food and Drug Act as amended (1938), and have been generally recognized among experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, as safe and effective for use under the conditions prescribed.” FDCA Section 201(p)1
Even without these new proposed rules, manufacturing, labeling, marketing, and sales of Homeopathic remedies would remain subject to FDA compliance rules. From 1982-1988, the industry, professional, and consumer members of the community through the American Homeopathic Pharmacists Association worked with the FDA in the development of a regulatory framework called Compliance Policy Guide. “The new CPG strengthened the definition of Homeopathic drugs, set forth guidelines for the prescription and non-prescription drugs, and made clear packaging and labeling guidelines.” This CPG was based on the law recognizing that even if Homeopathic remedies are sometimes called “Homeopathic Drugs,” there is a difference written into law. Under that law, drugs “shall be subject to the requirements of the United States Pharmacopeia unless it is labeled and offered for sale as a homeopathic drug, in which case it shall be subject to the provisions of the Homeopathic Pharmacopeia of the United States and not to those of the United States Pharmacopeia.”
Clearly, Homeopathic remedies have been and continue to be sufficiently regulated, by the FDA and by the Homeopathic industry itself. There has not been a single, verified case of harm or death from a Homeopathic remedy – in 200 years. There has not been a single case of addiction to Homeopathic remedies. Those are the facts, while chemical drug deaths contribute to the third leading cause of death in the nation – “accidental injury” – with drug overdose and the opioid crisis largely to blame. U.S. lifespan is falling, not despite pharmaceutical, chemical drugs, but because of them. Homeopathic remedies need to be accessible to those who opt to use them instead; it is not the role of the FDA to force one kind of medicine on American citizens to the exclusion of all others.
Categories Named by the FDA for New Regulation
In the FDA’s proposed Guidelines on Drug Products Labeled as Homeopathic, the FDA has proposed to withdraw the 1988 Compliance Policy Guide (lst revised in 1995) which governs the manufacturing and marketing of Homeopathic drugs.
The following categories, in bold type, are those targeted by the FDA for new regulation, with my comments below each category:
- Products with reported safety concerns.
There are no such products. A true and pure Homeopathic remedy as found in the HPUS cannot be anything but safe. The few products marketed as Homeopathic that have crept onto the marketplace with non-Homeopathic, synthetic ingredients including artificial colors, but which contain a low-potency “Homeopathic” ingredient, are a perversion of homeopathy (e.g. Zicam). The word “Homeopathic” could be removed from the labels of such products. Any pure Homeopathic product should be proven unsafe before the FDA can regulate it further, since according to the FDCA, Homeopathic drugs are generally recognized as safe, subject to the provisions of the HPUS. Homeopathy is an exact science, and one medicine cannot be used in place of another. If, for example, one has a Belladonna fever, no medicine other than Belladonna will help. Restricting any pure Homeopathic drugs found in the HPUS will cause needless suffering and hardship, and greater use and reliance on toxic pharmaceuticals. - Products that contain or purport to contain ingredients associated with potentially significant safety concerns. For example, potentially significant safety concerns are raised with products that contain or purport to contain: 1. an infectious agent with the potential to be pathogenic.
No true Homeopathic product could possibly be pathogenic. The words “associated with” pertain to the material substance, since no Homeopathic ingredient has any potential to be pathogenic. Critics say there is nothing in Homeopathic remedies, as, in fact, there is no measurable medicinal substance in them. Remedies made from disease products, called nosodes, or disease-causing matter (like Pyrogenium, made from decomposed beef) only sound risky due to the matter from which they’re derived; super-diluting beyond Avogadro’s scale renders them harmless. The dynamic effects of an energetic remedy are distinct, and opposite, from the physiological or poisonous effects of the material substance. Homeopathic nosodes and other such remedies have proven to be invaluable medicinal tools to practitioners and patients for centuries, their safety and benefit understood at the passage of the FDCA. - A controlled substance.
Although a few Homeopathic remedies are made from substances that are controlled in their material state (e.g., opium), the Homeopathic remedy made from opium is energy only. Homeopathically prepared opium cannot ‘drug’ the consumer, be lethal, nor addictive. It will leave the person with no effect at all, or, if needed, can direct the body’s energy to correct stupor or other symptoms associated with effects of the material drug. - Multiple Ingredients.
Homeopathic ingredients used in combination cannot harmfully interact with each other. Such combination remedies may be effective, if one of the ingredients matches symptoms of the individual’s illness. These can be beneficial to the consumer who does not have access to a professional homeopath to guide him/her to the best, single remedy. - Ingredients that pose potential toxic effects.
Homeopathic drugs are controlled in the manufacturing process, according to existing law. So long as that is accomplished, there is no potential for toxicity; pure Homeopathic drugs are merely energetic. Many valuable Homeopathic remedies happen to made from plants and other natural substances that can be toxic in material dose, which is of no consequence in assessing pure, Homeopathic/energetic drugs. - Products for routes of administration other than oral and topical.
Homeopathic drugs are properly used orally and topically. They are not intended for injection, and the FDA could properly regulate that unusual circumstance. However, it is not clear whether the FDA objects to eye drops. Certain Homeopathic ophthalmic products have a long history of safety and efficacy, and should be allowed as long as the remedies continue to be manufactured according to the HPUS standards for purity and safety. One such product, made by Similasan Corp. of Switzerland, is an eye drop product made according to the FDA’s Good Manufacturing Practices (GMP). There is no reason the product should be prohibited or be required to be treated as a pharmaceutical. - Products intended to be used for the prevention or treatment of serious and/or life-threatening diseases or conditions.
No true Homeopathic remedy or product is intended to prevent or treat a specific, named disease. Homeopathy treats an individual’s symptoms, not any disease. Remedy choice is based on characteristic (individual) symptoms. Labels can reflect this fact, and all Homeopathic medicines continue to be regulated according to the HPUS. - Products for vulnerable populations . . . such as immunocompromised individuals, infants and children, the elderly, and pregnant women . . . due to their varying ability to absorb, metabolize, distribute, or excrete the products or its metabolites.
Homeopathic remedies are completely safe, and do not need to be absorbed, metabolized, distributed, or excreted by the body and result in no metabolites. This is an especially disconcerting category named for greater regulation, since it is these very population groups that can gain the most from the use of non-toxic, energetic Homeopathic remedies that produce no side-effects, pose no toxicity, and cannot be addictive. - Products deemed adulterated under section 501 of the FD&C Act.
If a Homeopathic product purports to be or is represented as a product recognized in an official compendium but its strength, quality, or purity differs from the standard set forth in that official compendium (defined by 21 U.S.C. 321 as the official HPUS, National Formulary, or any supplement to any of them), or if there are significant violations of current good manufacturing practice requirements, then it should be judged accordingly, ideally by the Homeopathic governing body, the HPCUS. Medicines called “homeopathic” that contain synthetic ingredients like artificial color and flavor can be considered adulterated, in my opinion (even if the active ingredient is Homeopathic); they are not truly Homeopathic in purity. But this is an area covered by existing law; no new regulation is needed.
 laws on the books do not need to be amended and supplemented by unelected bureaucrats at the FDA. The original intent of Congress in passing the FDCA was not to promote a single, dominant system of healing in America, but instead, to allow for both chemical and Homeopathic medicines to be available to the public so long as labels disclose all of the ingredients and the remedies are pure, unadulterated, and made according to the pharmacopeia standards applicable to each. This new attack on homeopathy by Big Pharma and its friends must be defeated.
laws on the books do not need to be amended and supplemented by unelected bureaucrats at the FDA. The original intent of Congress in passing the FDCA was not to promote a single, dominant system of healing in America, but instead, to allow for both chemical and Homeopathic medicines to be available to the public so long as labels disclose all of the ingredients and the remedies are pure, unadulterated, and made according to the pharmacopeia standards applicable to each. This new attack on homeopathy by Big Pharma and its friends must be defeated.How to File Comments (NOTE: The period for filing comments has been extended to May 21, 2018.)
On March 21, 2017, the FDA extended the ruling period to May 21, 2018. The link to file your comments is https://www.regulations.gov/comment?D=FDA-2017-D-6580-3476.
Just as those who came before us have long defended Homeopathy from attack by politicians and competing schools of medicine, the job now falls to us. Frankly, it does not matter if you use Homeopathic remedies or products, or ever plan to. All should be concerned about arbitrary restrictions on the choice. But especially if you have used Homeopathy, make your voice heard today – the deadline is before Midnight on Monday, May 21, 2018.
Please click here to read some of the comments that were submitted before the initial deadline of March 20, 2018.
SAMPLE COMMENTS (You can “cut and paste” if you would like)
I strongly oppose any effort to impose new bureaucratic rules and regulations on Homeopathic remedies and products. The FDA has no authority to treat completely safe Homeopathic products as though they were toxic pharmaceutical chemicals. Millions of people in the United States and around the world have used Homeopathic products for more than two centuries without problem. Americans would not use these products if they were not helpful. Even though BIG PHARMA provides much of the FDA’s budget, it should not dictate its policies. The FDA must follow the law, and since 1938 Homeopathic remedies have been given a special status by Congress because they are not toxic and dangerous drugs. The American people insist on continued, unrestricted access to Homeopathic products.
*************
